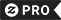Tap / click on image to see more RealViewsTM
$41.00
per shirt
IUPAC International Union Pure & Applied Chemistry T-Shirt
Qty:
Style
Basic T-Shirt
-$2.45
+$17.00
Colour & Print Process
White
Classic Printing: No Underbase
+$2.05
+$2.05
+$2.05
+$2.05
+$2.05
+$2.05
+$2.05
+$2.05
+$2.05
+$2.05
+$2.05
+$2.05
+$2.05
Vivid Printing: White Underbase
+$10.15
+$10.15
+$10.15
+$10.15
+$10.15
+$10.15
+$10.15
+$10.15
+$10.15
+$10.15
+$10.15
+$10.15
+$10.15
About T-Shirts
Sold by
About This Design
IUPAC International Union Pure & Applied Chemistry T-Shirt
The IUPAC nomenclature of organic chemistry is a systematic method of naming organic chemical compounds as recommended[1] by the International Union of Pure and Applied Chemistry (IUPAC). Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be drawn. There is also an IUPAC nomenclature of inorganic chemistry. See also phanes nomenclature of highly complex cyclic molecules. For ordinary communication, to spare a tedious description, the official IUPAC naming recommendations are not always followed in practice except when it is necessary to give a concise definition to a compound, or when the IUPAC name is simpler (e.g. ethanol against ethyl alcohol). Otherwise the common or trivial name may be used, often derived from the source of the compound In chemistry, a number of prefixes, suffixes and infixes are used to describe the type and position of functional groups in the compound. The steps to naming an organic compound are: 1.Identify the parent hydrocarbon chain. This chain must follow the following rules, in order of precedence: 1.It should have maximum substituents of the suffix functional group. By suffix, it is meant that the parent functional group should have a suffix, unlike halogen substituents. If more than one functional group is present, use the one with highest precedence as shown here. 2.It should have maximum number of multiple bonds 3.It should have maximum number of double bonds. 4.It should have the maximum length. 2.Identify the parent functional group, if any, with the highest order of precedence. 3.Identify the side-chains. Side chains are the carbon chains that are not in the parent chain, but are branched off from it. 4.Identify the remaining functional groups, if any, and name them by the name of their ions (such as hydroxy for -OH, oxy for =O, oxyalkane for O-R, etc.). Different side-chains and functional groups will be grouped together in alphabetical order. (The prefixes di-, tri-, etc. are not taken into consideration for grouping alphabetically. For example, ethyl comes before dihydroxy or dimethyl, as the "e" in "ethyl" precedes the "h" in "dihydroxy" and the "m" in "dimethyl" alphabetically. The "di" is not considered in either case). In the case of there being both side chains and secondary functional groups, they should be written mixed together in one group rather than in two separate groups. 5.Identify double/triple bonds. 6.Number the chain. To number the chain, first number in both directions (left to right and right to left), and then choose the numbering which follows these rules, in order of precedence: 1.Has the lowest locant (or locants) for the suffix functional group. Locants are the numbers on the carbons to which the substituent is directly attached. 2.Has the lowest locants for multiple bonds (The locant of a multiple bond is the number of the adjacent carbon with a lower number). 3.Has the lowest locants for double bonds 4.Has the lowest locants for prefixes. 7.Number the various substituents and bonds with their locants. If there is more than one of the same type of substituent/double bond, add the prefix (di-, tri-, etc.) before it. The numbers for that type of side chain will be grouped in ascending order and written before the name of the side-chain. If there are two side-chains with the same alpha carbon, the number will be written twice. Example: 2,2,3-trimethyl- . If there are both double bonds and triple bonds, write the "ene" before the "yne". In case the main functional group is a terminal functional group (A group which can only exist at the end of a chain, like formyl and carboxyl groups), there is no need to number it. 8.Arrange everything like this: Group of side chains and secondary functional groups with numbers made in step 3 + prefix of parent hydrocarbon chain (eth, ) + double/triple bonds with numbers (or "ane") + primary functional group suffix with numbers. Wherever it says "with numbers", it is understood that between the word and the numbers, you use the prefix(di-, tri-) 9.Add punctuation: 1.Put commas between numbers (2 5 5 becomes 2,5,5) 2.Put a hyphen between a number and a letter (2 5 5 trimethylheptane becomes 2,5,5-trimethylheptane) 3.Successive words are merged into one word (trimethyl heptane becomes trimethylheptane) Note: IUPAC uses one-word names throughout. This is why all parts are connected.
Customer Reviews
4.7 out of 5 stars rating31.8K Total Reviews
31,811 Reviews
Reviews for similar products
5 out of 5 stars rating
By Stuart B.28 June 2025 • Verified Purchase
Basic Dark T-Shirt, Black, Adult L
Excellent design platform, great quality material and printing, and quick delivery: couldn't ask for more...
5 out of 5 stars rating
By Shelley M.3 October 2022 • Verified Purchase
Value T-Shirt, White, Adult L
Zazzle Reviewer Program
I was really pleased with this shirt, the quality of the Tshirt was excellent, the artwork and print stands out and the fit is true to size....my son loved this for his 16th birthday 😀. Very good quality and color
5 out of 5 stars rating
By Vivienne C.9 June 2023 • Verified Purchase
Basic T-Shirt, White, Adult S
Zazzle Reviewer Program
AAA+++ Perfect gift for my old dad. He loved it - highly recommend and will buy again. Printing looked perfect to me
Tags
Other Info
Product ID: 235324414186965556
Posted on 6/05/2012, 11:32 AM
Rating: G
Recently Viewed Items