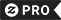Tap / click on image to see more RealViewsTM
$58.00
per mug
Which one will dissolve in water Polar Chemistry Travel Mug
Qty:
Style
Travel/Commuter Mug
-$23.25
-$20.90
-$18.60
-$11.60
-$9.30
-$4.65
Size
colour
Stainless Steel
About Mugs
Sold by
About This Design
Which one will dissolve in water Polar Chemistry Travel Mug
Polar bears are soluble in water. Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a liquid solvent to form a homogeneous solution. The solubility of a substance fundamentally depends on the used solvent as well as on temperature and pressure. The extent of the solubility of a substance in a specific solvent is measured as the saturation concentration where adding more solute does not increase the concentration of the solution. The solvent is generally a liquid, which can be a pure substance or a mixture.[1] One also speaks of solid solution, but rarely of solution in a gas (see vapour-liquid equilibrium instead) The extent of solubility ranges widely, from infinitely soluble (fully miscible[2] ) such as ethanol in water, to poorly soluble, such as silver chloride in water. The term insoluble is often applied to poorly or very poorly soluble compounds. Under certain conditions the equilibrium solubility can be exceeded to give a so-called supersaturated solution, which is metastable
Customer Reviews
4.8 out of 5 stars rating21.7K Total Reviews
21,687 Reviews
Reviews for similar products
5 out of 5 stars rating
By Anonymous27 August 2025 • Verified Purchase
Travel/Commuter Mug, 444 ml
Very happy with both the quality of the print and travel mug itself. Was hesitant being from NZ that the item wouldn't arrive in time for Father's day given the shipping guidelines, but it took only 5 days to arrive - 2 days before estimated delivery. Highly recommended. .
5 out of 5 stars rating
By Janette C.28 February 2016 • Verified Purchase
Travel/Commuter Mug, 444 ml
Zazzle Reviewer Program
Excellent well made, great gift for a man on the go. Good clear black writing. Very happy.
5 out of 5 stars rating
By Anonymous31 August 2025 • Verified Purchase
Travel/Commuter Mug, 444 ml
everything is still going great .
from zazzle.com (US)
Tags
Other Info
Product ID: 168632215263790115
Posted on 17/04/2010, 5:34 PM
Rating: G
Recently Viewed Items